Research
Viscosity-Structure Relationship of
Molten Slags and Glasses
(고온 세라믹 융체의 고온점성 측정 및 원자단위 구조와의 상관성 규명 연구 )
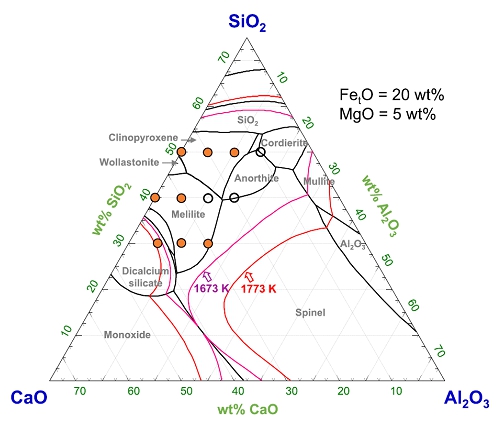
Influence of Al2O3 and SiO2 on the structure and viscosity of iron-compound bearing calcium-aluminosilicate slags
(Kim and Park; J. Alloys & Compd., 2022, vol. 916, article no. 165328)
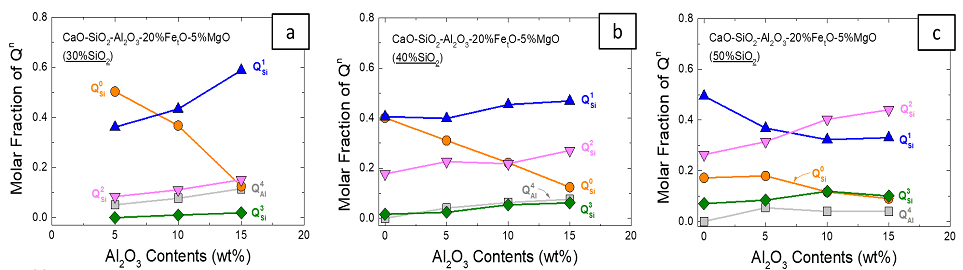
Viscosity-Structure Relationship of CaO-Al2O3-FetO-SiO2-MgO Ruhrstahl-Heraeus (RH) Refining Slag
(Kim and Park; ISIJ Int., 2021, vol. 61, pp. 724-733)
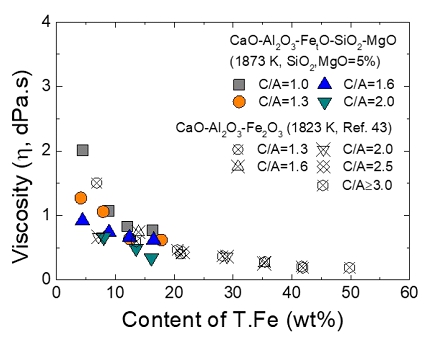
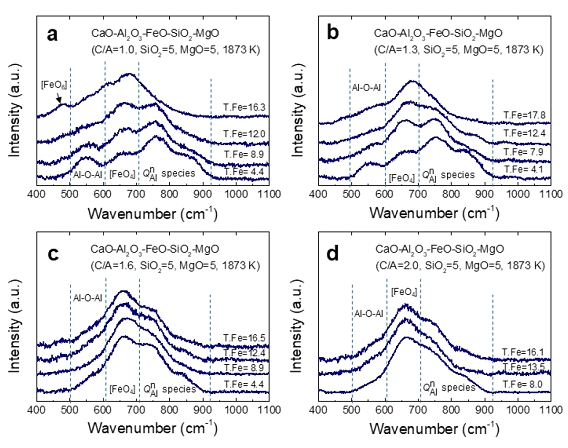
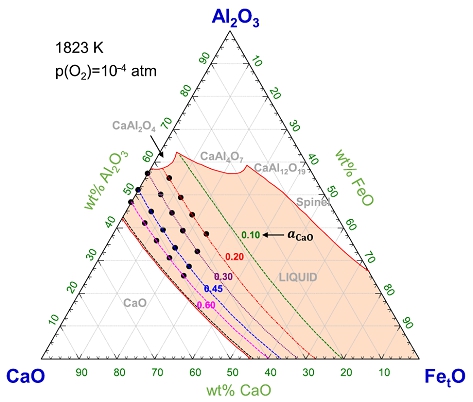
Thermodynamics of iron redox equilibria and viscosity-structure relationship of CaO-Al2O3-FetO melts
(Kim and Park; J. Non-Cryst. Solids, 2020, vol. 542, article no. 120089)
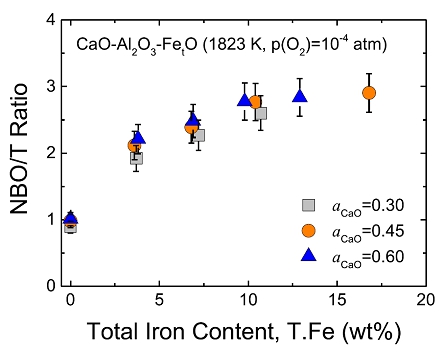
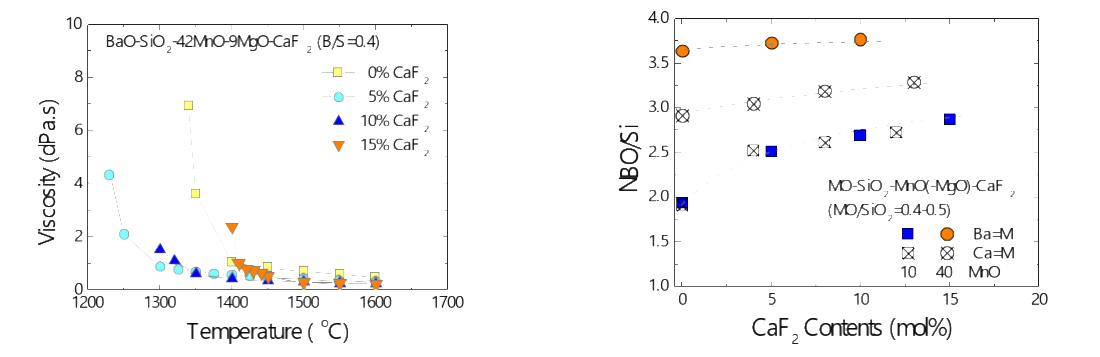
Viscosity-structure relationship of alkaline earth silicate melts containing manganese oxide and calcium fluoride
(Kim and Park: J. Am. Ceram. Soc., 2019, vol.102, pp.4943-4955)
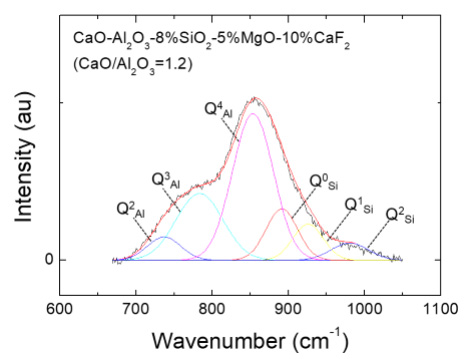
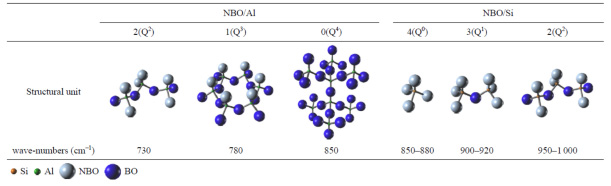
Structure-Viscosity Relationship of Low-silica Calcium Aluminosilicate Melts
(Kim and Park: ISIJ Int., 2014, vol.54, pp.2031-2038)
Structure-Property relationship of CaO-MgO-SiO2 system: Quantitative analysis of Raman spectra
(Park: Metall. Mater. Trans. B, 2013, vol.44B, pp.938-947) ; Marcus Grossmann Awarded paper [link]

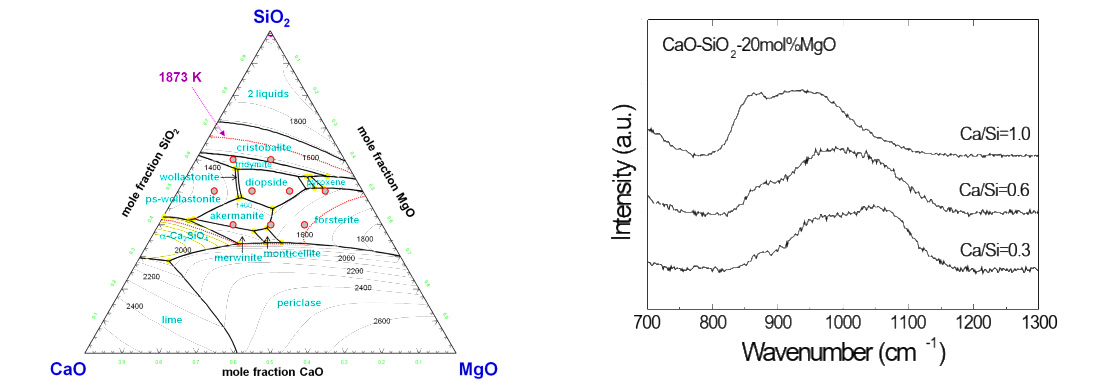
Effect of Silicate Structure on Thermodynamic Properties of Calcium Silicate Melts: Quantitative Analysis of Raman Spectra
(Park: Met. Mater. Int., 2013, vol.19, pp.577-584)
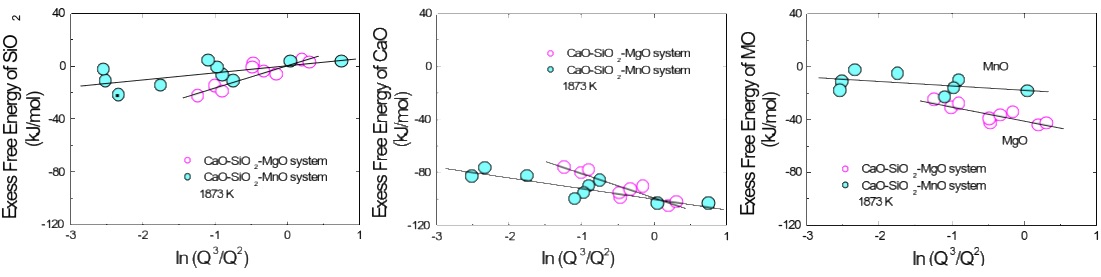
Composition-structure-property relationships of CaO-MO-SiO2 (M=Mg2+, Mn2+) systems derived from micro-Raman spectroscopy
(Park: J. Non-Cryst. Solids, 2012, vol.358, pp.3096-3102)
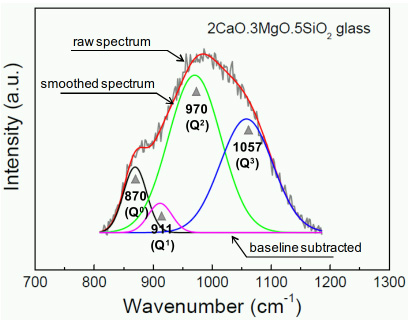
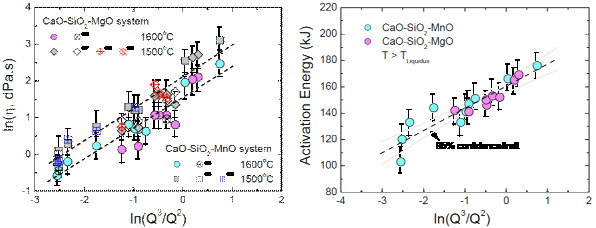
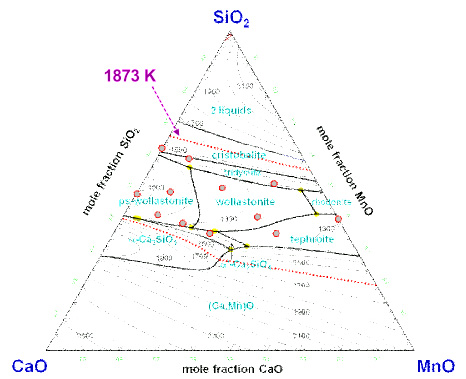
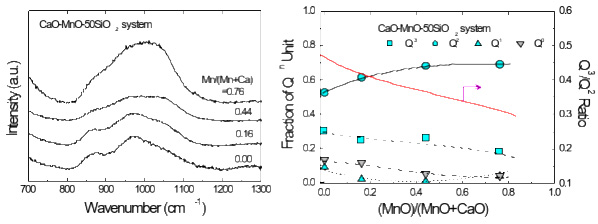
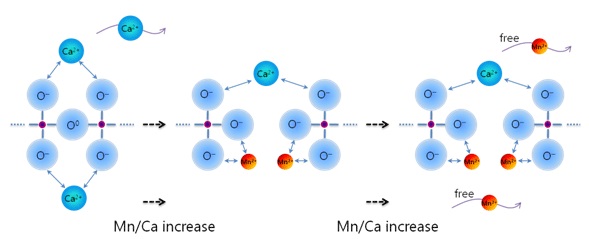
Structure–Property Correlations of CaO–SiO2–MnO Slag derived from Raman Spectroscopy
(Park: ISIJ Int., 2012, vol.52, pp. 1633-1642)
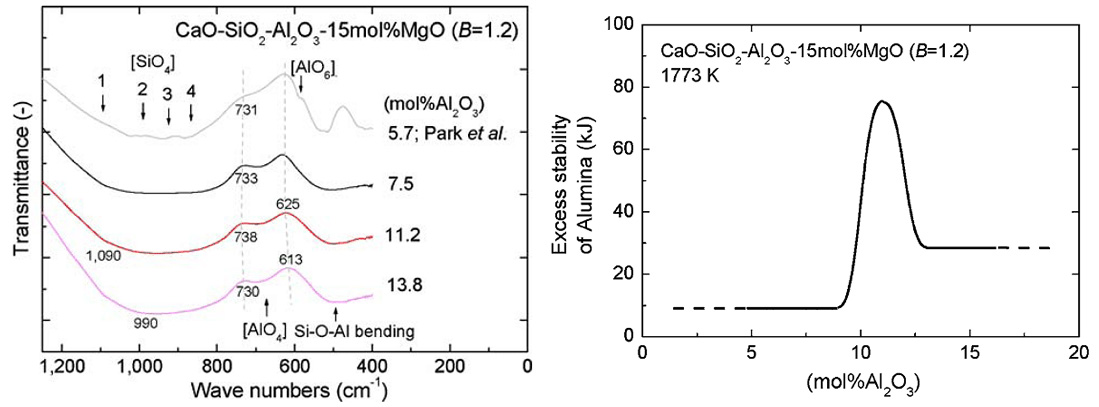
Novel Approach to Link between Viscosity and Structure of Silicate Melts via Darken's Excess Stability Function: Focus on the Amphoteric Behavior of Alumina
(Park, Kim, and Min; Metall. Mater. Trans. B, 2008, vol.39B, p.150)
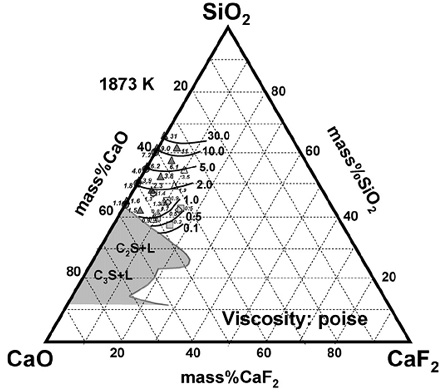
Structural Assessment of Fluorine in Calcium Silicates: Focusing on the Viscosity of CaO-SiO2-CaF2 System
(Park, Min, and Sasaki; ICS2008, October 2008, JAPAN; ISIJ Int., 2007, vol.47, p.1368)