Comparison of Oxidation Behavior of Various Reactive Elements in Alloys during Electroslag Remelting (ESR) Process: An Overview
(Duan and Park; ISIJ Int., 2022, vol. 62, pp. 1561-1572)
Electroslag remelting (ESR) is an advanced secondary refining technology to produce clean, fully dense, and homogeneous castings of steels and alloys by removal of undesirable elements and non-metallic inclusions. However, the alloying elements, such as Al/Ti, B/Si, rare earth metals (REM), in consumable electrode react with oxides in the slag during the ESR process, i.e., FeO, SiO2, TiO2, and Al2O3. Hence, the reactive elements cannot be maintained uniformly from the bottom to the top of the resultant ingots and subsequently inadequate mechanical properties of the metallic materials.
To date, the application of IMCT has also been further expanded to CaF2-containing slags used for the ESR process. FactSageTM software (Hereinafter referred to as thermodynamic calculation software) has been popularly applied in the iron- and steelmaking process to calculate some thermodynamic parameters and physicochemical properties of metallurgical melts. However, the accuracy of the calculated activities by the aforesaid methods has been rarely compared. In the present study, therefore, both the activities in CaF2 containing slags calculated by the IMCT model and the thermodynamic calculation software are used to compare each other and further investigate the effect of slag composition and temperature on the oxidation behavior of various reactive elements, such as Al/Ti, B/Si, and Ce(La)/Al, in alloy melts, which is to find a key parameter to restraint the oxidation loss during the ESR process.
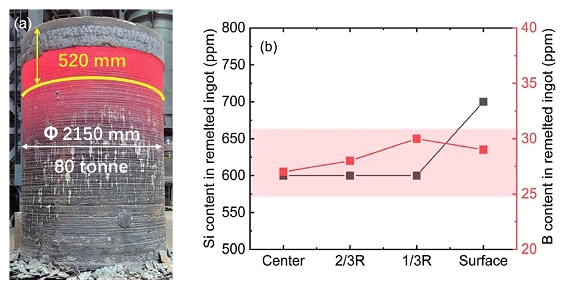
(a) Picture of remelted ESR ingot, and (b) Si and B content along with the radial direction of the remelted ingot.
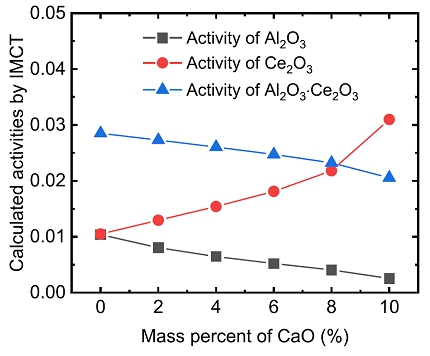
Relationship between calculated activities of Al2O3, Ce2O3 as well as Al2O3·Ce2O3 and CaO content in the CaF2–CaO–Al2O3–Ce2O3 at 1873 K
Influence of slag composition and oxygen potential on thermodynamic behavior of vanadium in FeO-TiO2-MgO-SiO2-Al2O3 smelting slag and molten iron
(Heo, Lee, HS Park and JH Park; J. Mater. Res. & Technol., 2021, vol. 15, pp. 5723-5732)
Vanadium has been defined as an important strategic metal that is used mainly as an alloying element in production of various steels and alloys in the ferrous and non-ferrous industries due to its beneficial physical properties, including high tensile strength, hardness, and fatigue resistance. The steelmaking industry is the primary market for vanadium consumption (approx. 85% in global), which has increased continuously with increases in steel production. In addition, the demand for vanadium in the aerospace and nuclear industries for production of non-ferrous vanadium alloys such as Ti-Al-V alloy and superalloys is expected to increase. Hence, the demand for vanadium is forecast to increase if demand for steel and alloys experiences steady growth.
Various studies have investigated the thermodynamics of vanadium oxide (VOx) in various types of CaO- and Na2O-based molten slags, but there are no reported studies of the fundamental thermodynamics of VOx and its valence variation in ilmenite smelting slag, i.e., FetO-TiO2 based slag system. In particular, evaluation of vanadium distribution has been performed mainly in carbon-saturated iron at high temperatures. In addition, ilmenite contains small amounts of impurities, such as Al2O3, SiO2, and MgO, which potentially affect the thermodynamic behavior of VOx as well as the Vox valence. Therefore, the present study endeavors to understand the effects of oxygen partial pressure and slag composition on the thermodynamic behavior of VOx in the FetO-TiO2-MgO-Al2O3-SiO2 slag equilibrated with molten iron. This research will elucidate fundamentals of the thermodynamic behavior of VOx during ilmenite smelting process.
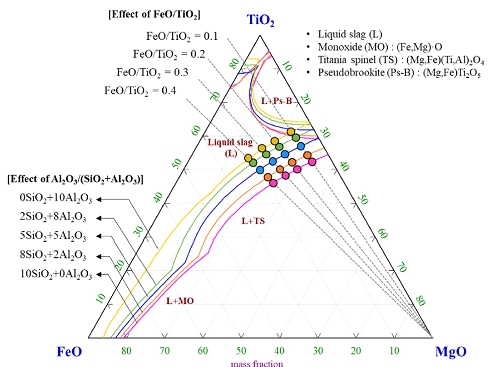
Experimental compositions of the FeO-TiO2-MgO-SiO2-Al2O3 (SiO2+Al2O3=10wt%) slag at 1550 oC and p(O2)=10-10 atm. The phase diagram was calculated by FactSageTM 7.3 software.
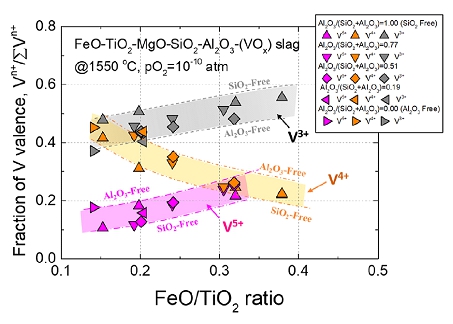
Effect of FeO/TiO2 ratio on the fraction of vanadium oxide valence at 1550 oC under p(O2)=10-10 atm.
Observations of FeO Reduction in Electric Arc Furnace Slag by Aluminum Black Dross: Effect of CaO Fluxing on Slag Morphology
(Heo, Kim, Sahajwalla and Park; Metall. Mater. Trans. B, 2020, vol. 51B, pp. 1201-1210)
Aluminum dross by-products of the aluminum industry are typically classified into three types based on their concentrations of metallic Al: aluminum white dross (AWD, 15-70 mass pct Al), aluminum black dross (ABD, 10-20 pct Al), and aluminum salt cake (ASC, approximately 5 pct Al). In particular, ABD is classified as hazardous waste according to the European Waste Catalogue and the United States Environmental Protection Agency’s Hazardous Waste List (entry 100309), because ABD has the following listed properties: highly flammable (H3-A), harmful (H5), toxic (H6), leachable (H13), and ecotoxic (H14). ABD reacts strongly with water, leaching out harmful and toxic gases such as NH3, CH4, PH3, and H2S. Thus, several physicochemical studies on the characterization of ABD have been carried out, including investigations of properties such as density, porosity, and toxicity as well as mineralogical and structural interpretations.
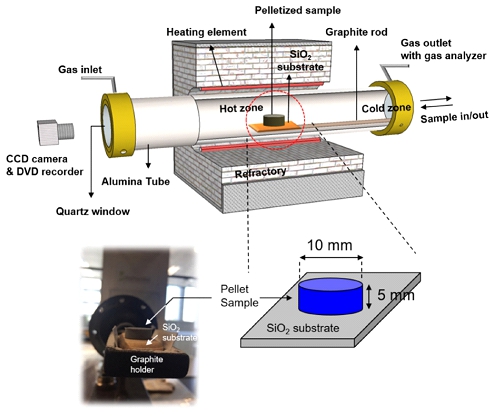
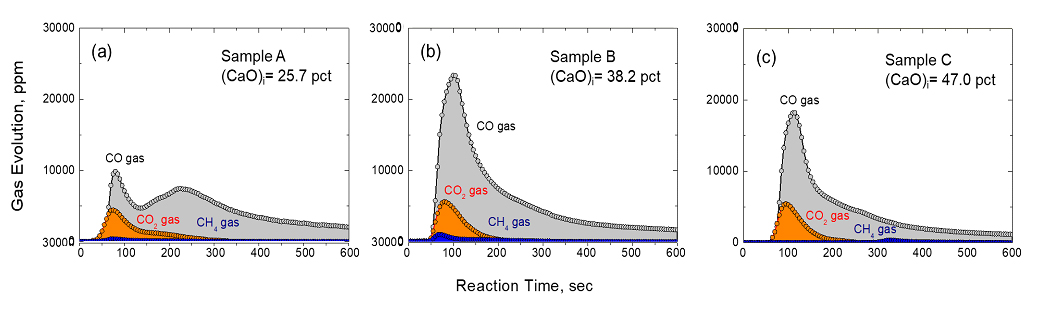
Even though the viscosity of molten slag during the reduction reaction was quantitatively evaluated, macroscopic observations have yet not been attempted to confirm how strongly the system is affected by slag viscosity. Therefore, as a follow-up to previous research, in the present work, we investigated the effect of CaO fluxing upon slag morphology during the reduction of FeO in EAF slag by ABD by in-situ observation of differences in pellet shape. This study will macroscopically confirm the importance of appropriate CaO fluxing of EAF slag when ABD is used as a reducing agent.
Schematic diagram of the experimental apparatus.
Evolution of gases (CO, CO2 and CH4) as a function of initial CaO content at various reaction times at 1773 K.
Assessment of Physicochemical Properties of Electrical Arc Furnace Slag and their Effects on Foamability
(Heo and Park; Metall. Mater. Trans. B, 2019, vol.50B, pp.2959-2968)
Slag foaming can be performed in an electric arc furnace (EAF). This has several advantages including protection of the metal bath, enhanced post combustion ratio, shielding of the electrical arc, reduced arc flares, and reduced erosion of the refractory in the furnace. Slag foaming can decrease electric power consumption by 3 to 10 pct in EAF operation because foamy slag contributes to post combustion and heat transfer, which are key parameters to an energy efficient process, as well as decreased refractory consumption by 25 to 63 pct.
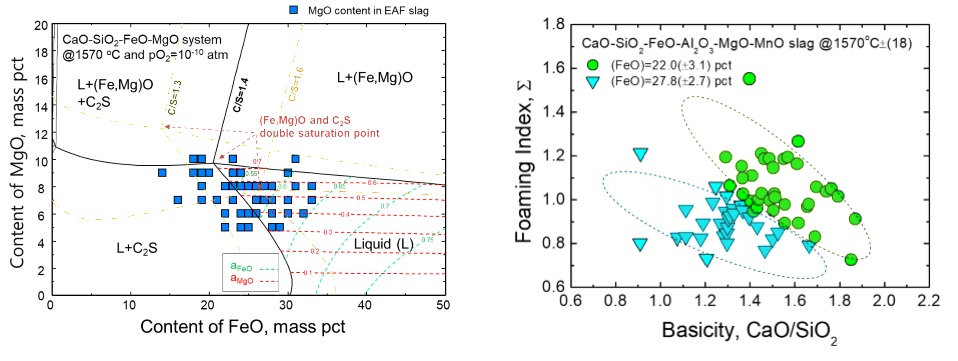
In summary, a number of research groups have studied foaming behavior by considering thermophysical properties of slags based on various principles such as dimensional analysis, phase equilibria, and structural analysis. However, a few studies have investigated slag foaming in the EAF process using thermodynamics and phase equilibria. Because slag foaming typically originates from carbothermic reduction, i.e., reaction between FeO in molten slag and injected carbon, understanding the behavior of FeO in molten slag in the EAF process is critically important. Therefore, in the present study, the EAF slag composition was considered to understand slag foaming characteristics using industrial EAF process data, from which the optimizing slag composition to obtain an efficient foamability was recommended.
Distribution of MgO in the CaO-SiO2-FeO-MgO (C/S = 1.4) phase diagram at 1843 K and pO2 = 10-5 Pa (=10-10 atm).
Effects of basicity and FeO content on the foaming index of EAF slags.
Effect of Direct Reduced Iron (DRI) on Dephosphorization of Molten Steel by Electric Arc Furnace Slag
(Heo and Park; Metall. Mater. Trans. B, 2018, vol.49B, pp.3381-3389) ; TMS-EPD
(Extraction & Processing Division) Science Awarded Paper [link]
The use of direct reduced iron (DRI) as a substitute for premium scraps in electric arc furnace (EAF) steelmaking has increased in popularity because DRI does not have tramp elements, has a uniform density and shape, as well as higher yield and purity of carbon, affords convenience of charging, and has lower cost of raw materials. Unfortunately, however, commercially available DRI contains a relatively high level of phosphorus, which adversely affects the properties of steels. The increased use of DRI to produce high-quality steels in an EAF also increases the likelihood of phosphorus contamination of the steel. Thus, phosphorus should be fully controlled in the EAF process. However, because there are various types of oxides (e.g., FetO, SiO2, Al2O3, CaO) as well as carbon present in DRI, a comprehensive understanding of the effect of DRI on slag formation behavior, and thus dephosphorization efficiency in the EAF process, is required.
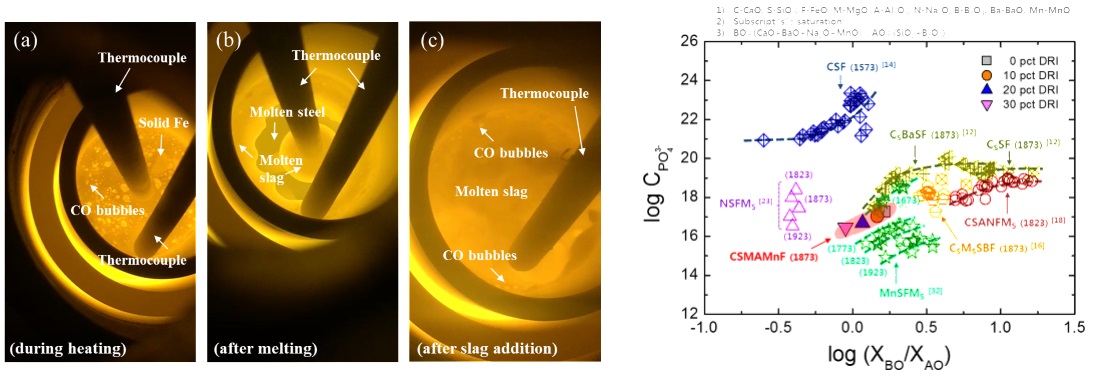
Even though numerous studies have investigated the thermodynamics of phosphorus on a laboratory scale and several plant EAF trials have used DRI, our fundamental understanding of phosphorus behavior when using DRI is incomplete. In particular, the effects of various phenomena such as the carborthermic reaction between FeO and carbon in DRI, slagmaking by gangue melting in DRI, and oxygen transfer from slag produced by gangue melting to molten steel on the behavior of phosphorus during DRI melting still have to be clarified. Variation in slag composition is caused by the presence of gangue in DRI, which has a critical impact on dephosphorization efficiency. Therefore, in the present study, we investigated the effect of DRI on dephosphorization reaction between molten steel and EAF slag, and discussed the phosphorus partition behavior based on experimental results and thermodynamic investigation.
Snapshot for reaction steps: (a) during heating, (b) after DRI melting, and (c) after slag addition.
Phosphate capacity as a function of basicity index, log (XBO/XAO), at different DRI contents.
Effect of slag chemistry on desulfurization kinetics in secondary refining process
(Kang, Shin, Chung and Park; Metall. Mater. Trans. B, 2017, vol.48B, pp.2123-2135)
In the production of bearing steel, it is important to ensure that the level of nonmetallic inclusions is as low as possible, especially for inclusions larger than 15 um, since the inclusions can potentially initiate cracks under severe conditions. It is well known that sulfur is easily precipitated at the grain boundary in the form of sulfide inclusions due to a low solubility in the austenite and ferrite matrix. Consequently, the mechanical properties of steel become degraded.
Although many studies exist with regard to desulfurization kinetics, the bulk of them have focused on desulfurization rates under strong stirring conditions. Additionally, there are insufficient studies related to the desulfurization reaction mechanism, which is strongly affected by the physicochemical properties of the slag. Finally, the experimental composition range of slag, which has been investigated, is relatively limited. Consequently, in the present study, we focused on desulfurization behavior according to a wide composition of slag considering plant conditions such as slag compositions and working temperatures. Moreover, the RCS for desulfurization during ladle refining processes and the transition of the RCS through slag composition will be systematically discussed.
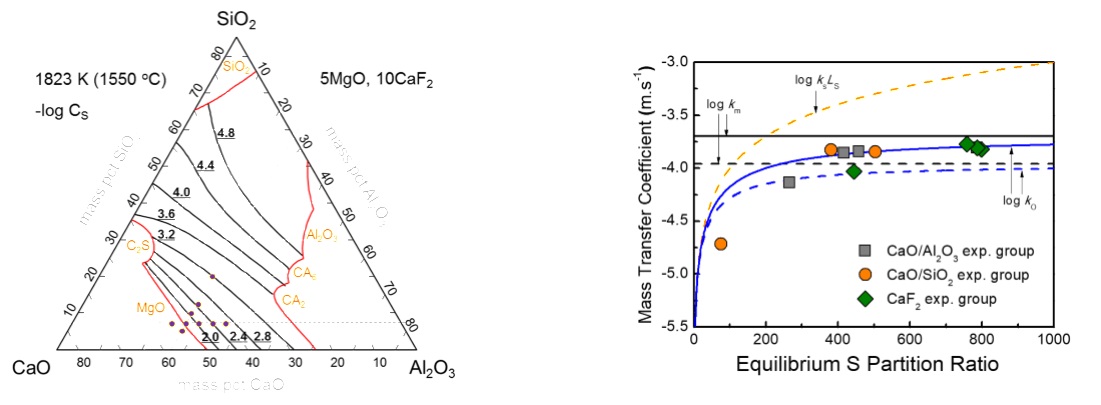
Experimental composition of the present study represented in the CaO-Al2O3-SiO2-5MgO-10CaF2 (mass pct) phase diagram at 1823 K, calculated by FACTSAGE 7.0.
Relationship between the mass-transfer coefficients and equilibrium S partition ratio (solid lines are from present study, and dashed lines are from the literature).
Relationship between sulfide capacity and structure of MnO-SiO2-Al2O3-Ce2O3 system
(Jeong, Kim and Park; Metall. Mater. Trans. B, 2017, vol.48B, pp.545-553)
It is well known that sulfur is harmful to the mechanical properties of steel product such as strength, ductility, toughness. Also, sulfide or oxy-sulfide inclusions deteriorate the corrosion resistance and oxidation resistance of stainless steels, and thus it is desirable that these harmful inclusions should be effectively removed. However, sulfur is contained as an impurity in various alloying materials such as ferroalloys. The formation of rare earth sulfide or oxy-sulfide inclusions in molten steel is unavoidable due to high affinity between rare earth elements and sulfur at steelmaking temperatures. Therefore, the sulfur absorption ability of the oxide system containing rare earth elements should be necessarily investigated at high temperatures.
In the present study, we measured the sulfide capacity of the MnO-SiO2-Al2O3-Ce2O3 quaternary system by gas-slag equilibration method at 1873 K, and the structural analysis was also carried out using micro-Raman spectroscopic method to understand the role of Ce2O3 in the sulfur dissolution behavior in the present oxide system because none of thermodynamic information (activity of each component, phase equilibria, etc.) of the MnO-SiO2-Al2O3-Ce2O3 system is available.
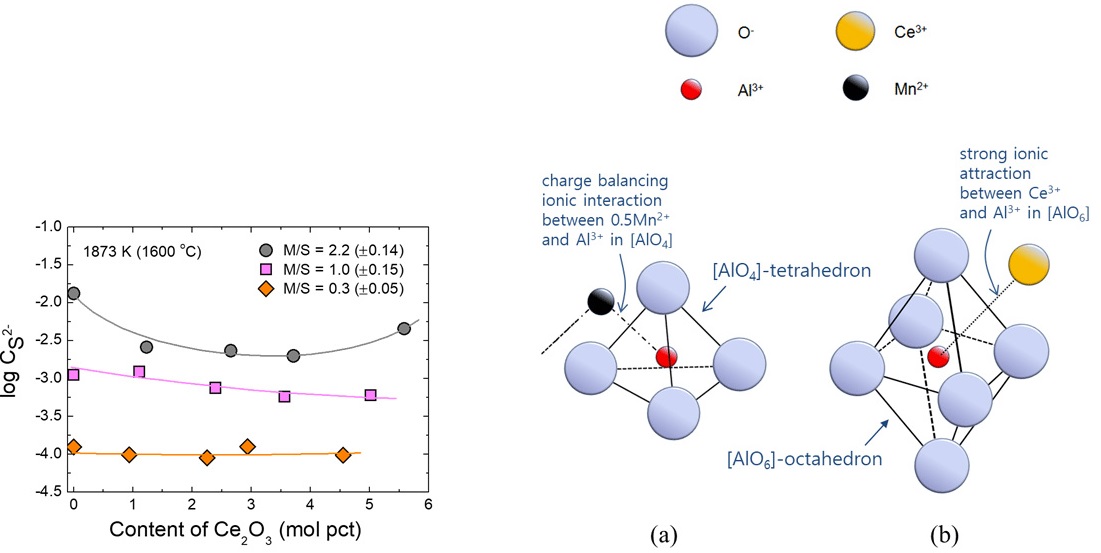
Effect of Ce2O3 on the sulfide capacity of the MnO-SiO2-Al2O3-Ce2O3 system with different MnO/SiO2 ratios at 1873 K.
Schematics of the ionic structure of (a) [AlO4]-tetrahedron balanced with 0.5Mn2+ ion (hypothetically shown as [(Al,Mn0.5)O4]-unit) and (b) [AlO6]-octahedron balanced with Ce3+ ion (hypothetically shown as [(Al,Ce)O6]-unit).
Thermodynamics of Reducing Refining of Phosphorus from Si-Mn Alloy using CaO-CaF2 Slag
(Shin and Park; Metall. Mater. Trans. B, 2012, vol.43B, pp.1243-1246)
High manganese steels (HMnS, 10~25%Mn) are of interest due to their good mechanical properties including superior strength and good ductility. Accordingly, the demand for ultra low phosphorus manganese alloys such as FeMn and SiMn alloys has recently increased. However, the conventional oxidizing dephosphorization technique is not applicable to manganese alloys because silicon or manganese will be oxidized before phosphorus under oxidizing conditions. Therefore, the dephosphorization should be carried out under a strongly reducing atmosphere to produce a low phosphorus Si-Mn alloy.
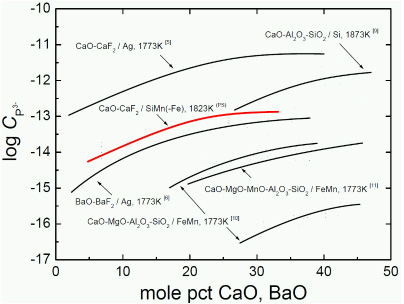
Although several studies concerning the phosphide capacity of lime-based flux systems have been published, there are few reports of reducing refining of phosphorus from Si-Mn alloys. Hence, in the present study, we investigated the thermodynamic behavior of phosphide ions in the CaO-CaF2 flux in equilibrium with a Si-Mn alloy melt under a strongly reducing atmosphere.
Phosphide capacity for the various flux systems as a function of molar content of basic oxide (PS; present study).
Effect of Slag Composition on the Distribution Behavior of Pb between FetO-SiO2 (-CaO, Al2O3) Slag and Molten Copper
(Heo and Park; Metall. Mater. Trans. B, 2012, vol.43B, pp.1098-1105)
It is very important in copper smelting and refining processes to remove several minor elements such as Pb, Bi, Sb, As, and Te. Specifically, Pb is a troublesome impurity which increases the amount of slime in the electro-refining process. Also, from the viewpoint of copper smelting and refining processes, Pb is a detrimental element that is always present in sulfide concentrates and must be eliminated. Therefore, the content of Pb in molten copper should be lowered within an appropriate concentration range during plant operation; thus, we need specific knowledge of the effect of slag composition on the thermodynamic behavior of Pb in molten slag.
Even though the effectiveness of slag treatment for the removal of Pb from the Cu melt has been investigated as mentioned above, many studies focused on the equilibrium distribution behavior of Pb in the Cu/matte/slag system during the smelting and converting processes. Therefore, in the present study, the distribution behavior of Pb between molten copper and FetO-SiO2 (-CaO, Al2O3) slags was investigated from the view of the reaction mechanism of Pb dissolution into the slag. Furthermore, the lead capacity of the slag was estimated from the experimental results.
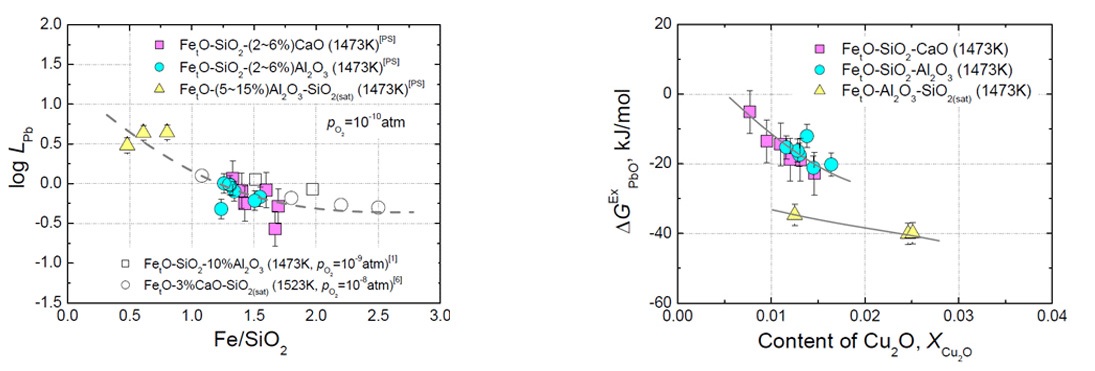
Influence of Fe/SiO2 ratio on log LPb in the FetO-SiO2 (-CaO, Al2O3) slags at 1473 K (1200 oC) and log p(O2)=-10. [PS]: present study.
Excess free energy of PbO in the FetO-SiO2 (-CaO, Al2O3) slags at 1473 K (1200 oC).
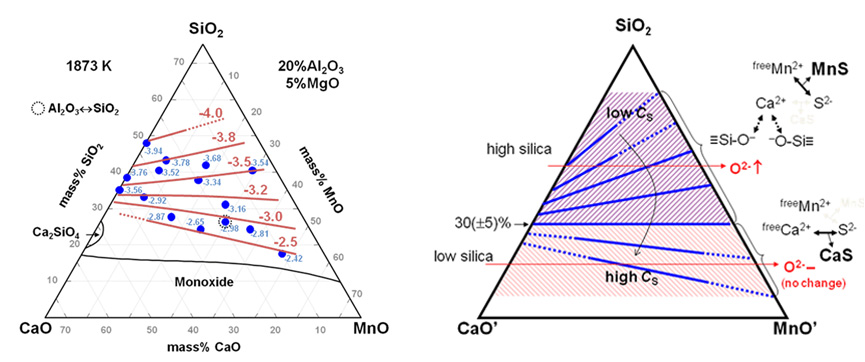
Sulfide Capacity of the CaO-SiO2-MnO-Al2O3-MgO Slag at 1873 K
(Park et al.; ISIJ Int., 2012, vol.52, pp. 764-769)
Desulfurization has been emphasized over several decades in iron- and steelmaking processes. Sulfur is harmful to the mechanical properties of steel products (e.g., strength, ductility and toughness). Therefore, it is necessary to remove sulfur from molten steel to under several ppm in order to improve mechanical properties. Additionally, the demand for ferrous and non-ferrous manganese alloys has increased continuously due to the introduction of advanced high strength steels such as transformation induced plasticity (TRIP) and twining induced plasticity (TWIP) aided steels that were recently developed to contain manganese up to about 30 mass%. Thus, the desulfurization of manganese (ferro-)alloys and high manganese steels is an important issue.
Even though many researchers have investigated the sulfide capacities of molten slags, there are few experimental data regarding high MnO-containing multicomponent slags. We recently investigated experimental and modeling approaches for the CaO-SiO2-MnO ternary system. In the present paper, we focus on sulfur dissolution behavior into multicomponent calcium manganosilicate melts containing Al2O3 and MgO.
Therefore, in the present study we measured the sulfide capacities of the CaO-SiO2-MnO-Al2O3-5 mass% MgO slags at 1873 K through a wide composition range, and also discussed the thermodynamic effects of basicity and the stability of sulfide on the dissolution behavior of sulfur into the CaO-SiO2-MnO-Al2O3-MgO slags.
Iso-sulfide capacity contour in the CaO-SiO2-MnO-20%Al2O3-5%MgO slag at 1873 K.
Concept of “Competitive dissolution mechanism of Sulfur” in the CaO-MnO-SiO2-base slags.
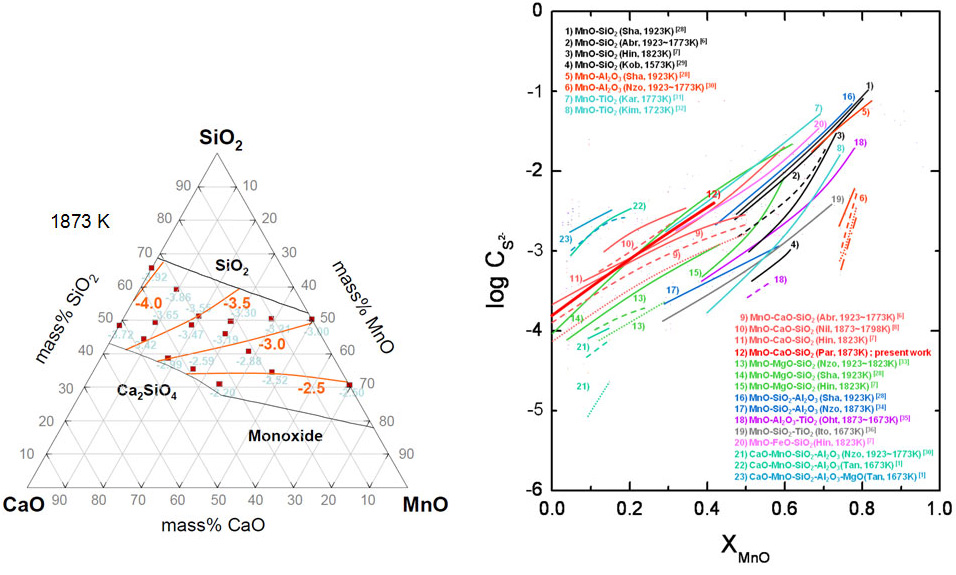
Sulfide Capacity of the CaO-SiO2-MnO Slag at 1873 K
(Park et al.; ISIJ Int., 2011, vol.51, pp.1375-1382)
In the steelmaking process, the importance of desulfurization has been emphasized during several decades. It is well known that the sulfur is harmful to the mechanical properties of steel products such as strength, ductility and toughness, etc. Therefore, it is necessary to remove the sulfur from molten steel under several ppm in order to improve the mechanical properties of steel products. Also, the demand of ferrous and non-ferrous Mn alloys is continuously increasing because the advanced high strength steels such as TRIP and TWIP steels which were recently developed contain Mn up to about 30 mass%. Therefore, the desulfurization of Mn (ferro–)alloys as well as high Mn steels has recently been issued.
Even though many researchers have investigated the sulfide capacity of molten slags, there are a few experimental data for the CaO–SiO2–MnO ternary system at 1873 K. Therefore, it is needed not only to measure the sulfide capacity of the CaO–SiO2–MnO slag at 1873 K through the entire composition range but also to discuss the thermodynamic effect of basicity and the stability of sulfide on the dissolution behavior of sulfur into the CaO–SiO2–MnO slag.
On the other hand, Sosinsky and Sommerville proposed the correlation between the sulfide capacity of various slags and the optical basicity by incorporating the temperature effect. This relationship has been widely used not only in academia but also in industries during last decades. They determined the effective (or empirical) optical basicity for several transition metal oxides including MnO, however the effective optical basicity for MnO (Ls,MnO = 1.2) was greater than the theoretical value (LMnO = 1.0) originally calculated from Pauling’s electronegativity of cation by Duffy et al. Hence, some researchers have been confused to employ the optical basicity concept to predict the sulfide capacity of the MnO–containing slags.
Therefore, in the present study, the sulfide capacity of the CaO–SiO2–MnO slag through the entire composition range was measured at 1873 K using a gas–slag equilibration method and the effect of basicity and the activity coefficient of sulfide on the sulfide capacity of molten slag was investigated. Furthermore, the relationship between the sulfide capacity of MnO–containing slags and the optical basicity was evaluated in view of industrial applications.
Iso-sulfide capacity contour in the CaO-SiO2-MnO slag at 1873 K.
Sulfide capacities of the various slag systems containing MnO as a function of MnO content.
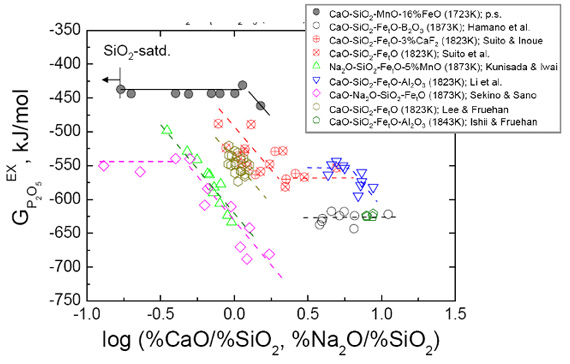
Phosphate Capacity of CaO-SiO2-MnO-FeO Slag saturated with MgO
(Cho, Park, and Min; ISIJ Int., 2010, vol.50, p. 324)
Phosphate capacity of the lime-based slags has widely been investigated by numerous authors since Wagner originaly introduced the phosphate capacity concept as a measure of the ability of a slag to absorb phosphorus. However, according to the polymerization degree of phosphate ions, the equilibrium reaction between monomer (PO43-, orthophosphate) and dimer (P2O74-, pyrophosphate) was proposed. An orthophosphate ion has been known to be stabilized at a content of phosphorus lower than a critical concentration in slag, e.g. 2%P in the CaO-SiO2-CaF2 slag doubly saturated with CaO and 3CaO×SiO2, and 1%P in the same slag saturated with 2CaO×SiO2 at 1573 K.
Almost of the previous studies regarding the phosphate capacity as well as the stable species of phosphate ions in the lime-based slags have mainly been carried out in the highly basic compositions, e.g. CaO saturation conditions, in view of hot metal treatment or steelmaking processes. Recently, however, because an interst on the phosphorus removal from various ferro-alloys, e.g. ferromanganese, has increased, the phosphate capacity of the smelting slag saturated with MgO, which is more acidic than general steelmaking fluxes, is widely necessiated.
Therefore, in the present study, the phosphate capacity of the relatively acidic lime-based slag saturated with MgO was investigated at 1723 K. The effect of basicity on the phosphate capacity and the stability of phosphate ions (ortho- or pyrophosphate) in the slag was especially focused.
Relationship between excess free energy of P2O5 and basicity index of various MgO-saturated slags. All slags are saturated with MgO. (p.s.; present study)
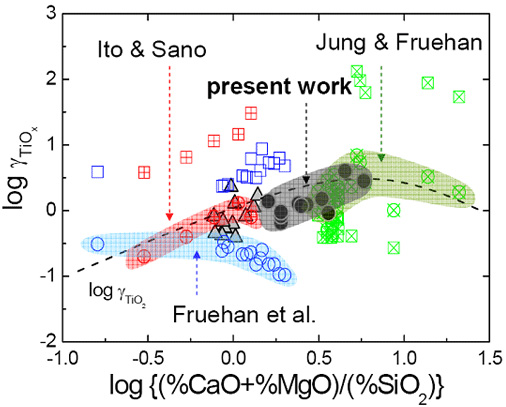
Thermodynamics of Titanium Oxide in CaO-SiO2-Al2O3-MgOsat-CaF2 Slag equilibrated with Fe-11%Cr Melt
(Park, Lee, Kim, and Pak; ISIJ Int., 2009, vol.49, p.337)
Titanium stabilized (stainless) steels have been the subject of previous studies related to clogging of the submerged entry nozzle (SEN) during continuous casting process. In view of high yield of Ti during ladle treatment, the thermodynamic behavior of Ti oxide in the slag is also very important. Even though there are numerous studies in redox equilibria of Ti in glasses and geochemical silicate melts, the thermodynamic behavior of Ti oxide in metallurgical slag system has not so clearly been understood yet. The almost of TiOx could be estimated as TiO2 at oxygen partial pressure determined by CO-CO2 equilibrium. From the previous studies, the effect of slag composition on the thermodynamic behavior of Ti oxide in the CaO-SiO2-Al2O3-MgO-TiOx slags has not been clarified yet.
Consequently, in the present work, the thermodynamic equilibrium between the CaO-SiO2-Al2O3-MgOsatd-5%CaF2(-TiOx) slags and the Fe-11%Cr melt was investigated at 1873 K in order to understand the thermodynamic behavior of Ti oxide in the refining slags.
Basicity dependence of the activity coefficient of TiO2 (and Ti2O3 and Ti3O5) in various slags.